Benefits of Laboratory Information Management Systems (LIMS) You Should Know
Software that helps laboratories worldwide with data administration and regulatory issues is known as a LIMS, or Laboratory Information administration System. It is utilized to handle samples and related data, standardizing reporting processes. The LIMS system was created to monitor sample transportation within a laboratory. Since then, LIMS has developed into a solution for data management requirements, including monitoring the results of tests. A configuration tool has also been developed as a feature. Tools like chemical software that match terminology and do not require customization enable the system to satisfy workflow requirements.
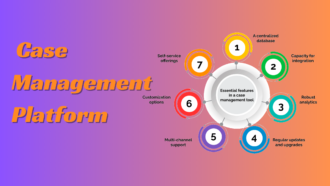
Laboratory Information Management Systems (LIMS)
Removing human error

When manually recording readings, measurements, and sample information, even the most attentive and meticulous lab worker is susceptible to error. These inaccuracies compound with increasing data volume, decreasing the accuracy and significance of your total results. Lab instrument integration with LIMS enables precise, automated, and quick reading acquisition. A LIMS offers a straightforward, paperless platform where results are available to any department that wants them immediately and can be examined as necessary, even when observations must be manually entered.
It Exchanges Data Automatically

A LIMS database is used to store the data. One of the main advantages of LIMS is the data flow across companies and departments. The system becomes data-driven and aids in improved decision-making by exchanging papers and information. The management software’s automated information interchange enhances clinic procedures and internal operations. By utilizing this program, you may expedite various activities and incorporate reporting time for various periods. Data visualization tools may display sample status information in the data.
Improved Efficiency

Improved operational and laboratory efficiency is one of the most essential benefits of laboratory information management systems. Your facility may become paperless by automating laboratory processes using LIMS, which maintains data integrity and boosts production. Information may be retrieved from a laboratory information management system, and false reporting and data loss can be prevented. Additionally, LIMS discourages you from doing analytical testing with out-of-date and out-of-calibrated equipment, raising your test findings’ caliber and accuracy. By doing away with manual procedures, you save turnaround time. Your personnel will have more time to devote to the primary duties of the company as a result.
Supports Regulatory Compliance

The laboratory information management system aids in ensuring that your business and personnel adhere to all applicable laws, rules, and standards. For example, the Food & Drug Administration (FDA) mandates an audit trail to keep track of all lab activity. Every time the company adds, edits, or removes a record, these actions must be recorded. You may comply with FDA regulatory requirements using the laboratory information management system to preserve a read-only audit trail. It continuously and automatically records all lab activity. The laboratory information management system uses electronic signatures to confirm crucial laboratory processes, assisting your company in conforming with 21 CFR Part 11 regulations.
The operational effectiveness of a laboratory is increased overall by the use of chemical software. A LIMS offers an effective data management solution by saving time that would otherwise be spent on manual data logging and maintenance. As a result, there are fewer human errors and more accurate data systems to support different judgments. Additionally, it has an audit trail, which automatically cuts down on the time required for human audits. A LIMS is also most helpful when dealing with large amounts of data that need batch processing and routine daily tasks. Additionally, it addresses numerous compliance requirements, assisting laboratories in maintaining their legal,health, and privacy standards.